Rat BMP2 ELISA Kit
- SKU:
- RTFI00008
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Uniprot:
- P49001
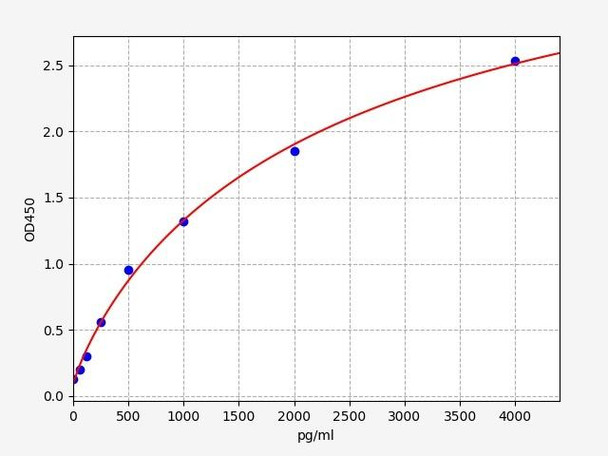
- Sensitivity:
- 37.5pg/ml
- Range:
- 62.5-4000pg/ml
- ELISA Type:
- Sandwich ELISA, Double Antibody
- Synonyms:
- BMP-2, Bone Morphogenetic Protein 2, BMP2A, BMP-2A
- Reactivity:
- Rat
Description
| 商品名: | Rat BMP-2 (Bone Morphogenetic Protein 2) ELISA Kit |
| 製品コード: | RTFI00008 |
| サイズ: | 96 Assays |
| 目標: | Rat BMP-2 |
| エイリアス: | BMP-2, Bone Morphogenetic Protein 2, BMP2A, BMP-2A |
| 反応性: | Rat |
| 検出方法: | Sandwich ELISA, Double Antibody |
| 感度: | 37.5pg/ml |
| 範囲: | 62.5-4000pg/ml |
| 保管所: | 4°C for 6 months |
| ノート: | For Research Use Only |
| 回復: | Matrices listed below were spiked with certain level of Rat BMP-2 and the recovery rates were calculated by comparing the measured value to the expected amount of Rat BMP-2 in samples. | ||||||||||||||||
| |||||||||||||||||
| 直線性: | The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Rat BMP-2 and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected. | ||||||||||||||||
| |||||||||||||||||
| Intra-Assay: | CV <8% | ||||||||||||||||
| Inter-Assay: | CV <10% |
| Uniprot: | P49001 |
| UniProt Protein Function: | BMP2: Induces cartilage and bone formation. Belongs to the TGF-beta family. |
| UniProt Protein Details: | Protein type:Motility/polarity/chemotaxis; Secreted, signal peptide; Secreted Cellular Component: extracellular space; protein complex; cell surface; cytoplasm; extracellular region Molecular Function:protein domain specific binding; protein homodimerization activity; growth factor activity; protein heterodimerization activity; phosphatase activator activity; cytokine activity; SMAD binding; transforming growth factor beta receptor binding; receptor binding; retinol dehydrogenase activity Biological Process: activation of MAPK activity; positive regulation of apoptosis; heart development; positive regulation of transcription, DNA-dependent; adrenocorticotropin hormone secreting cell differentiation; telencephalon regionalization; protein amino acid phosphorylation; cardiac muscle cell differentiation; regulation of apoptosis; BMP signaling pathway; ovulation cycle; chondrocyte differentiation; inner ear development; regulation of odontogenesis of dentine-containing teeth; positive regulation of astrocyte differentiation; pericardium development; positive regulation of neurogenesis; cell fate commitment; organ morphogenesis; mesenchymal cell differentiation; response to mechanical stimulus; regulation of MAPKKK cascade; positive regulation of osteoblast proliferation; positive regulation of fat cell differentiation; positive regulation of endothelial cell proliferation; positive regulation of transcription from RNA polymerase II promoter; negative regulation of transcription, DNA-dependent; positive regulation of cell differentiation; negative regulation of calcium-independent cell-cell adhesion; positive regulation of odontogenesis; proteoglycan metabolic process; embryonic heart tube anterior/posterior pattern formation; negative regulation of insulin-like growth factor receptor signaling pathway; thyroid stimulating hormone secreting cell differentiation; negative regulation of transcription from RNA polymerase II promoter; bone mineralization; negative regulation of cell cycle; negative regulation of cell proliferation; regulation of transcription, DNA-dependent; positive regulation of MAPKKK cascade; negative regulation of aldosterone biosynthetic process; cardiac muscle morphogensis; inflammatory response; positive regulation of Wnt receptor signaling pathway; cardiac cell differentiation; Notch signaling pathway; ossification; response to retinoic acid; protein destabilization; in utero embryonic development; positive regulation of bone mineralization; positive regulation of ossification; odontogenesis of dentine-containing teeth; osteoblast differentiation; positive regulation of osteoblast differentiation; telencephalon development; ureteric bud branching; cartilage development; response to hypoxia; epithelial to mesenchymal transition; positive regulation of protein amino acid phosphorylation; positive regulation of neuron differentiation; growth; positive regulation of cell migration |
| NCBI Summary: | involved in cellular signaling during limb development; induces bone formation [RGD, Feb 2006] |
| UniProt Code: | P49001 |
| NCBI GenInfo Identifier: | 1345612 |
| NCBI Gene ID: | 29373 |
| NCBI Accession: | P49001.1 |
| UniProt Related Accession: | P49001 |
| Molecular Weight: | 44,383 Da |
| NCBI Full Name: | Bone morphogenetic protein 2 |
| NCBI Synonym Full Names: | bone morphogenetic protein 2 |
| NCBI Official Symbol: | Bmp2 |
| NCBI Protein Information: | bone morphogenetic protein 2; BMP-2; BMP-2A; bone morphogenetic protein 2A |
| UniProt Protein Name: | Bone morphogenetic protein 2 |
| UniProt Synonym Protein Names: | Bone morphogenetic protein 2A; BMP-2A |
| Protein Family: | Bone morphogenetic protein |
| UniProt Gene Name: | Bmp2 |
| UniProt Entry Name: | BMP2_RAT |
| ステップ | プロトコル |
| 1. | Set standard, test sample and control (zero) wells on the pre-coated plate respectively, and then, record their positions. It is recommended to measure each standard and sample in duplicate. Wash plate 2 times before adding standard, sample and control (zero) wells! |
| 2. | Aliquot 0.1ml standard solutions into the standard wells. |
| 3. | Add 0.1 ml of Sample / Standard dilution buffer into the control (zero) well. |
| 4. | Add 0.1 ml of properly diluted sample ( Human serum, plasma, tissue homogenates and other biological fluids.) into test sample wells. |
| 5. | Seal the plate with a cover and incubate at 37°C for 90 min. |
| 6. | Remove the cover and discard the plate content, clap the plate on the absorbent filter papers or other absorbent material. Do NOT let the wells completely dry at any time. Wash plate X2. |
| 7. | Add 0.1 ml of Biotin- detection antibody working solution into the above wells (standard, test sample & zero wells). Add the solution at the bottom of each well without touching the side wall. |
| 8. | Seal the plate with a cover and incubate at 37°C for 60 min. |
| 9. | Remove the cover, and wash plate 3 times with Wash buffer. Let wash buffer rest in wells for 1 min between each wash. |
| 10. | Add 0.1 ml of SABC working solution into each well, cover the plate and incubate at 37°C for 30 min. |
| 11. | Remove the cover and wash plate 5 times with Wash buffer, and each time let the wash buffer stay in the wells for 1-2 min. |
| 12. | Add 90 µL of TMB substrate into each well, cover the plate and incubate at 37°C in dark within 10-20 min. (Note: This incubation time is for reference use only, the optimal time should be determined by end user.) And the shades of blue can be seen in the first 3-4 wells (with most concentrated standard solutions), the other wells show no obvious color. |
| 13. | Add 50 µL of Stop solution into each well and mix thoroughly. The color changes into yellow immediately. |
| 14. | Read the O.D. absorbance at 450 nm in a microplate reader immediately after adding the stop solution. |
ELISAアッセイを実施する場合、可能な限り最良の結果を達成するためにサンプルを準備することが重要です。以下に、さまざまなサンプルタイプのサンプルを準備するための手順のリストを示します。
| サンプルタイプ | プロトコル |
| 血清 | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clotovernight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Removeserum and assay promptly or aliquot and store the samples at-80°C. Avoid multiple freeze-thaw cycles. |
| プラズマ | Collect plasma using EDTA or heparin as an anti-coagulant. Centrifuge samples at 4°C for 15 mins at 1000 × g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles.Note: Over haemolysed samples are not suitable for use with this kit. |
| 尿および脳脊髄液 | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| 細胞培養上清 | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| 細胞溶解物 | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20°C. |
| 組織ホモジネート | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenizein 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or-80°C. |
| 組織溶解物 | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| 母乳 | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |