Anti-Phospho-PRKAA2 (S491) Antibody (RACO0116)
- SKU:
- RACO0116
- Product type:
- Recombinant Antibody
- Reactivity:
- Human
- Host Species:
- Human
- Isotype:
- IgG
- Application:
- WB
- Application:
- ELISA
- Conjugation:
- Unconjugated
Description
| 商品名: | Phospho-PRKAA2 (S491) Antibody |
| Product SKU: | RACO0116 |
| サイズ: | 50ul |
| 宿主種: | Human |
| 申し込み: | ELISA, WB |
| 推奨される希釈: | WB:1:500-1:5000 |
| 反応性: | Human |
| 免疫原: | A synthesized peptide derived from human Phospho-PRKAA2 (S491) |
| 憲法: | Liquid |
| ストレージバッファ: | Rabbit IgG in phosphate buffered saline , pH 7.4, 150mM NaCl, 0.02% sodium azide and 50% glycerol. |
| 精製方法: | Affinity-chromatography |
| 抗体のクローン性: | Monoclonal |
| アイソタイプ: | Rabbit IgG |
| Conjugate: | Non-conjugated |
| バックグラウンド: | Catalytic subunit of AMP-activated protein kinase (AMPK), an energy sensor protein kinase that plays a key role in regulating cellular energy metabolism. In response to reduction of intracellular ATP levels, AMPK activates energy-producing pathways and inhibits energy-consuming processes: inhibits protein, carbohydrate and lipid biosynthesis, as well as cell growth and proliferation. AMPK acts via direct phosphorylation of metabolic enzymes, and by longer-term effects via phosphorylation of transcription regulators. Also acts as a regulator of cellular polarity by remodeling the actin cytoskeleton; probably by indirectly activating myosin. Regulates lipid synthesis by phosphorylating and inactivating lipid metabolic enzymes such as ACACA, ACACB, GYS1, HMGCR and LIPE; regulates fatty acid and cholesterol synthesis by phosphorylating acetyl-CoA carboxylase (ACACA and ACACB) and hormone-sensitive lipase (LIPE) enzymes, respectively. Regulates insulin-signaling and glycolysis by phosphorylating IRS1, PFKFB2 and PFKFB3. Involved in insulin receptor/INSR internalization (PubMed:25687571). AMPK stimulates glucose uptake in muscle by increasing the translocation of the glucose transporter SLC2A4/GLUT4 to the plasma membrane, possibly by mediating phosphorylation of TBC1D4/AS160. Regulates transcription and chromatin structure by phosphorylating transcription regulators involved in energy metabolism such as CRTC2/TORC2, FOXO3, histone H2B, HDAC5, MEF2C, MLXIPL/ChREBP, EP300, HNF4A, p53/TP53, SREBF1, SREBF2 and PPARGC1A. Acts as a key regulator of glucose homeostasis in liver by phosphorylating CRTC2/TORC2, leading to CRTC2/TORC2 sequestration in the cytoplasm. In response to stress, phosphorylates 'Ser-36' of histone H2B (H2BS36ph), leading to promote transcription. Acts as a key regulator of cell growth and proliferation by phosphorylating TSC2, RPTOR and ATG1/ULK1: in response to nutrient limitation, negatively regulates the mTORC1 complex by phosphorylating RPTOR component of the mTORC1 complex and by phosphorylating and activating TSC2. In response to nutrient limitation, promotes autophagy by phosphorylating and activating ATG1/ULK1. AMPK also acts as a regulator of circadian rhythm by mediating phosphorylation of CRY1, leading to destabilize it. May regulate the Wnt signaling pathway by phosphorylating CTNNB1, leading to stabilize it. Also phosphorylates CFTR, EEF2K, KLC1, NOS3 and SLC12A1. Plays an important role in the differential regulation of pro-autophagy (composed of PIK3C3, BECN1, PIK3R4 and UVRAG or ATG14) and non-autophagy (composed of PIK3C3, BECN1 and PIK3R4) complexes, in response to glucose starvation. Can inhibit the non-autophagy complex by phosphorylating PIK3C3 and can activate the pro-autophagy complex by phosphorylating BECN1 (By similarity). |
| シノニム: | 5'-AMP-activated protein kinase catalytic subunit alpha-2, Acetyl-CoA carboxylase kinase, PRKAA2, AMPK, AMPK2 |
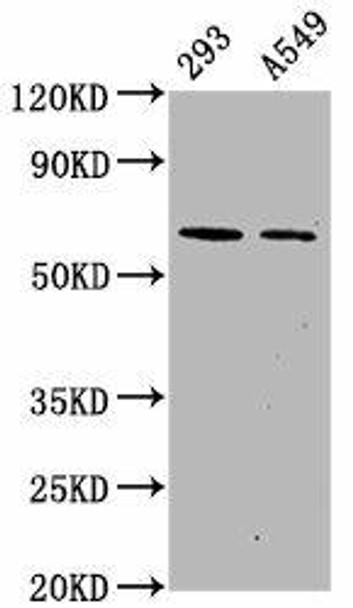
 | Western Blot. Positive WB detected in 293 whole cell lysate A549 whole cell lysate All lanes Phospho-PRKAA2 antibody at 1 (1µg) ml. Secondary. Goat polyclonal to rabbit IgG at 1:50000 dilution. Predicted band size: 62 KDa. Observed band size: 62 KDa. |
| UniProt Protein Function: | AMPKA2: a catalytic subunit of AMP-activated protein kinase (AMPK). Acts as an energy sensor, playing a key role in regulating cellular energy metabolism. A protein kinase of the CAMKL family whose activation is regulated by the balance between ADP/AMP/ATP, and intracellular Ca(2+) levels. Acts as a metabolic stress-sensing protein kinase switching off biosynthetic pathways when cellular ATP levels are depleted and when 5'-ADP and -AMP rise in response to fuel limitation and/or hypoxia. Activates energy-producing pathways and inhibits energy-consuming processes. Restores ATP levels in cells by switching off anabolic and switching on catabolic pathways. Activated primarily by rising ADP levels and not, as previously thought, solely by AMP. AMPK resembles an adenylate charge regulatory system in which anabolic and catabolic pathways are regulated by adenine nucleotide ratios. Acts via direct phosphorylation of metabolic enzymes and transcription regulators. Regulates fatty acid synthesis by phosphorylating acetyl-CoA carboxylase. Regulates cholesterol synthesis by phosphorylating and inactivating hormone-sensitive lipase and hydroxymethylglutaryl-CoA reductase. Activated by at least two distinct upstream kinases: the tumor suppressor LKB1 and CaMKK2. Also acts as a regulator of cellular polarity by remodeling the actin cytoskeleton, probably by indirectly activating myosin. AMPK is a heterotrimer of an alpha catalytic subunit (AMPKA1 or -2), a beta (AMPKB1 or -2) and a gamma non-catalytic subunit (AMPKG1, -2 or -3). Different possible combinations of subunits give rise to 12 different holoenzymes. Binding of ADP or AMP to non-catalytic gamma subunit (PRKAG1, -2 or -3) results in allosteric activation. AMPK is activated by antihyperglycemic drug metformin, a drug prescribed to patients with type 2 diabetes: in vivo, metformin seems to mainly inhibit liver gluconeogenesis. However, metformin can be used to activate AMPK in muscle and other cells in culture or ex vivo. Selectively inhibited by compound C (6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyyrazolo[1,5-a] pyrimidine. Activated by resveratrol, a natural polyphenol present in red wine, and S17834, a synthetic polyphenol. Salicylate/aspirin directly activates kinase activity. Studies in the mouse suggest that AMPK2 may control whole-body insulin sensitivity and is necessary for maintaining myocardial energy homeostasis during ischemia. |
| UniProt Protein Details: | Protein type:EC 2.7.11.31; EC 2.7.11.1; Protein kinase, Ser/Thr (non-receptor); Autophagy; Kinase, protein; Protein kinase, CAMK; EC 2.7.11.27; CAMK group; CAMKL family; AMPK subfamily Chromosomal Location of Human Ortholog: 1p31 Cellular Component: AMP-activated protein kinase complex; cytoplasm; cytosol; nucleoplasm Molecular Function:[acetyl-CoA carboxylase] kinase activity; [hydroxymethylglutaryl-CoA reductase (NADPH)] kinase activity; AMP-activated protein kinase activity; ATP binding; chromatin binding; histone serine kinase activity; metal ion binding; protein binding; protein kinase activity; protein serine/threonine kinase activity; protein serine/threonine/tyrosine kinase activity Biological Process: carnitine shuttle; cell cycle arrest; cellular lipid metabolic process; cellular response to glucose starvation; cellular response to nutrient levels; cholesterol biosynthetic process; energy reserve metabolic process; fatty acid biosynthetic process; fatty acid homeostasis; gene expression; glucose homeostasis; insulin receptor signaling pathway; lipid biosynthetic process; macroautophagy; mitochondrion organization and biogenesis; negative regulation of apoptosis; negative regulation of TOR signaling pathway; organelle organization and biogenesis; positive regulation of autophagy; positive regulation of glycolysis; positive regulation of macroautophagy; protein amino acid phosphorylation; regulation of circadian rhythm; regulation of fatty acid biosynthetic process; regulation of macroautophagy; regulation of transcription, DNA-dependent; response to muscle activity; response to stress; rhythmic process; signal transduction; transcription initiation from RNA polymerase II promoter; Wnt receptor signaling pathway |
| NCBI Summary: | The protein encoded by this gene is a catalytic subunit of the AMP-activated protein kinase (AMPK). AMPK is a heterotrimer consisting of an alpha catalytic subunit, and non-catalytic beta and gamma subunits. AMPK is an important energy-sensing enzyme that monitors cellular energy status. In response to cellular metabolic stresses, AMPK is activated, and thus phosphorylates and inactivates acetyl-CoA carboxylase (ACC) and beta-hydroxy beta-methylglutaryl-CoA reductase (HMGCR), key enzymes involved in regulating de novo biosynthesis of fatty acid and cholesterol. Studies of the mouse counterpart suggest that this catalytic subunit may control whole-body insulin sensitivity and is necessary for maintaining myocardial energy homeostasis during ischemia. [provided by RefSeq, Jul 2008] |
| UniProt Code: | P54646 |
| NCBI GenInfo Identifier: | 20178276 |
| NCBI Gene ID: | 5563 |
| NCBI Accession: | P54646.2 |
| UniProt Secondary Accession: | P54646,Q9H1E8, Q9UD43, |
| UniProt Related Accession: | P54646,AAB32732 |
| Molecular Weight: | 62,320 Da |
| NCBI Full Name: | 5'-AMP-activated protein kinase catalytic subunit alpha-2 |
| NCBI Synonym Full Names: | protein kinase AMP-activated catalytic subunit alpha 2 |
| NCBI Official Symbol: | PRKAA2 |
| NCBI Official Synonym Symbols: | AMPK; AMPK2; PRKAA; AMPKa2 |
| NCBI Protein Information: | 5'-AMP-activated protein kinase catalytic subunit alpha-2 |
| UniProt Protein Name: | 5'-AMP-activated protein kinase catalytic subunit alpha-2 |
| UniProt Synonym Protein Names: | Acetyl-CoA carboxylase kinase (EC:2.7.11.27 |
| UniProt Gene Name: | PRKAA2 |
| UniProt Entry Name: | AAPK2_HUMAN |